Research Summary
Ability to Consent to Clinical Trials in Males and Females with Fragile X Syndrome
Date Published: October 2019
What was the research about?
Researchers learn about possible new treatments for fragile X syndrome (FXS) through clinical trials. To take part in a clinical trial, adults and adolescents need to sign a form to give consent or assent. These forms can be hard to understand. Our Fragile X World researchers wanted to learn how well individuals with FXS understand what it means to take part in a clinical trial. We studied the ability to consent in individuals with FXS. Understanding the ability to consent of individuals with FXS can help researchers improve the consent process. They may be able to give better information and support to help people decide whether to take part in a clinical trial. It can also help those with FXS be more informed about their health care decisions.
What did the research team do?
We made changes to a well-known clinical interview that has been used to measure the ability to consent. We created a story where a character with FXS was invited to take part in a pretend clinical trial by his doctor. We read participants the story on a flip chart. The story had basic information about the trial, such as what the trial was about, what it would be like if they took part, and the risks and benefits of being in the trial. The flip chart included simple words and pictures.
The researcher read the flip chart to each participant and then asked open-ended questions to determine their ability to consent. The questions measured each participant's:
- ability to understand the information presented,
- ability to appreciate how taking part in the trial would affect their own care,
- ability to select reasons for wanting or not wanting to take part in the trial, and
- ability to make a choice about taking part.
If the participant didn't get the open-ended question right on the first try, the researcher showed the participant the information again. Then the participant got a second try to answer the question. On the second try, participants answered multiple-choice questions instead of open-ended questions about the story.
Participants also completed surveys and activities to measure their IQ, memory, and autism status. These are areas that may affect their ability to consent.
Parents of participants completed surveys about their child's anxiety, adaptive behavior, and social communication. These are other areas which may affect ability to consent.
Who was in the study?
A total of 152 individuals with FXS, ages 12-40, took part in the study. About 53% of the participants were males and 47% were females. The average age of both males and females was around 20 years old. More males (38%) had a co-diagnosis of autism than females (7%). The participants lived all over the U.S., 89% were white, and 70% reported a family income of $75,000 or more a year.
Figure 1a. Males with FXS with and without autism
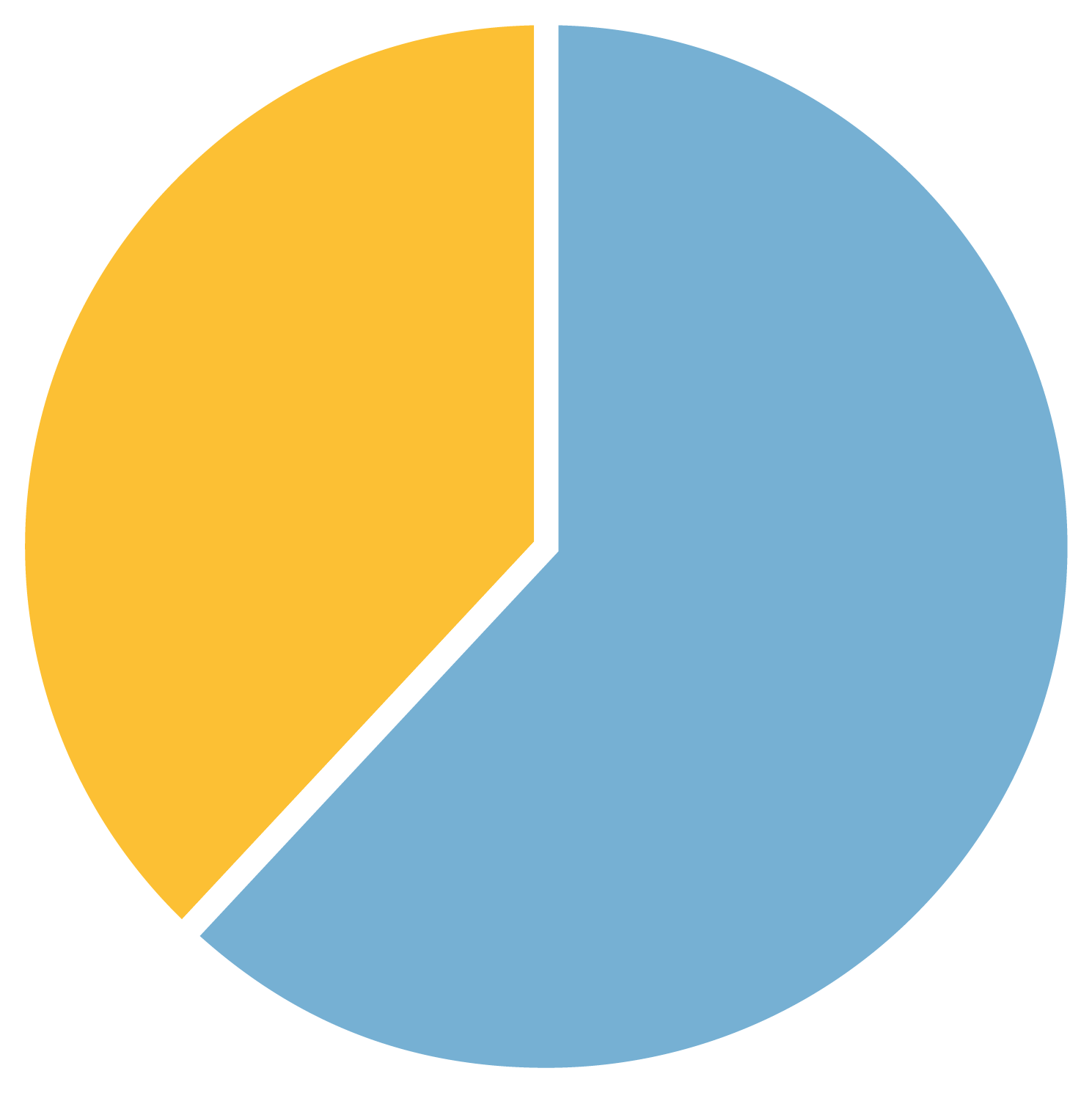
38% Males with FXS and autism
20 years
average age of males with FXS included in the study
Figure 1b. Females with FXS with and without autism
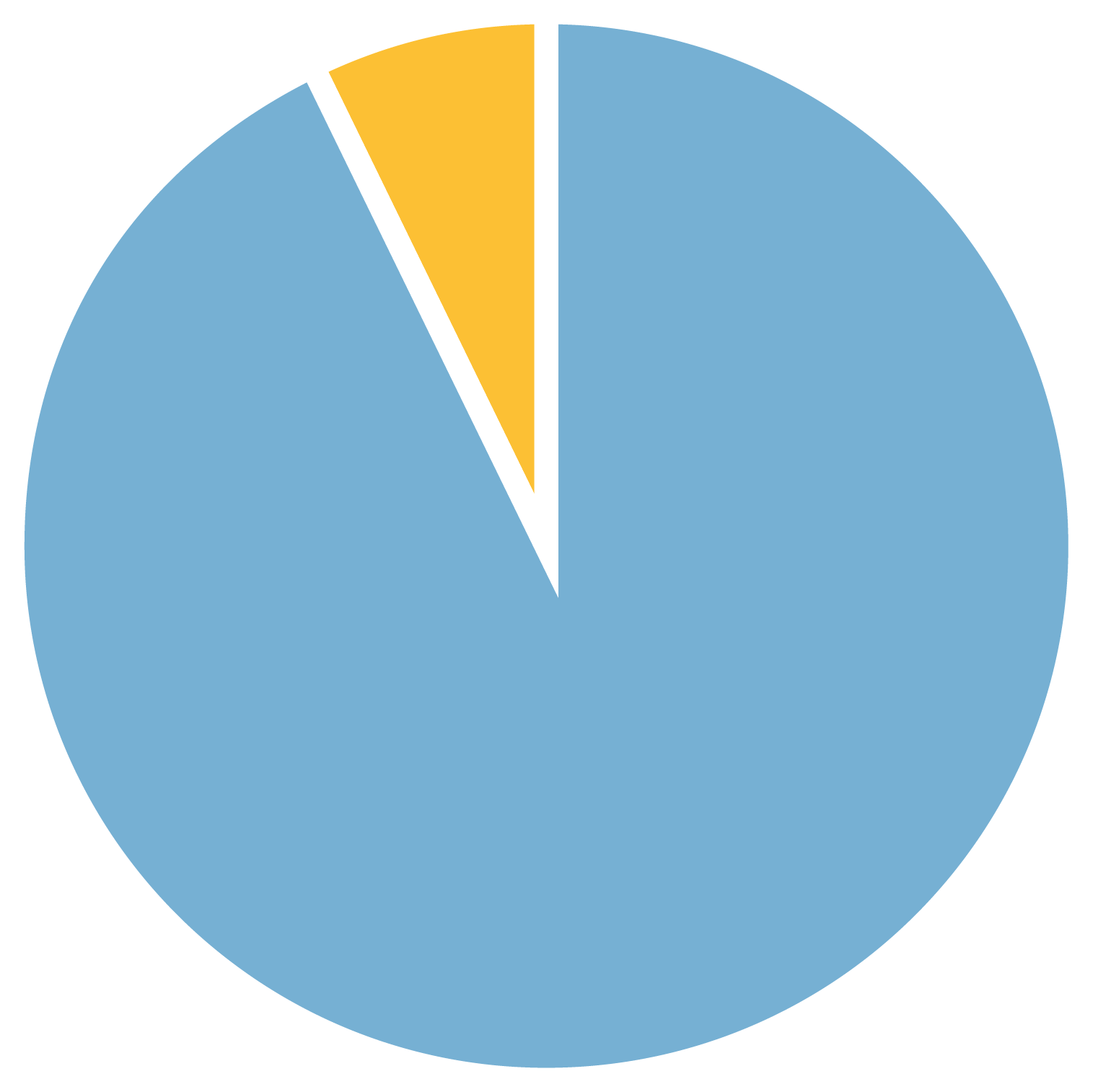
7% Females with FXS and autism
21 years
average age of females with FXS included in the study
What did the research team learn?
Females had better capacity to consent than males, but their profiles were similar.
Females showed higher levels of ability to consent than males. However, males and females had similar strengths and weakness within the four areas:
- Understanding: This area was where both males and females performed best. Participants were able to understand trial information like what the participant would need to do in the clinical trial, how they may benefit, and how often they would need to take the study medicine. However, abstract concepts like placebo and randomization were more difficult to understand. This was true even after repeating the information and giving a second try to answer the questions. Figure 2 and Figure 3 below provide information about which concepts were easier or harder for males and females to understand.
- Appreciation: Both males and females had trouble appreciating how their care would be different if they took part in the trial. When given a second try with multiple-choice answers, their appreciation improved a little.
- Reasoning: This area was also difficult for both males and females. Both groups did not understand that taking part in research was not the same as receiving regular clinical care.
- Expressing a choice: Most females were able to make a choice without support (79%). But only about half of the males (49%) made a choice at first. When the researchers repeated the information and gave multiple-choice options, this improved. With a second try, 81% of the males and 96% of the females were able to make a choice about whether to take part in the pretend clinical trial.
Participant characteristics had to do with ability to consent
Other characteristics were important in predicting ability to understand the information about the clinical trial. Participants with higher IQ, oral comprehension, and memory had a better ability to consent. Participants who had lower anxiety also had better ability to consent. These characteristics were more important than the age, sex, or autism status of the individual with FXS.
Figure 2. Understanding of clinical trial concepts among males with FXS
Note: Percentages do not total to 100% due to rounding.
Source: Our Fragile X World
| First try | Second try | Never correct | |
|---|---|---|---|
| Doctor visit | 65% | 23% | 12% |
| Risk | 65% | 16% | 19% |
| Duration of study | 60% | 16% | 24% |
| Placebo | 7% | 20% | 73% |
| Randomization | 21% | 32% | 46% |
| Societal benefit | 8% | 51% | 41% |
Figure 3. Understanding of clinical trial concepts among females with FXS
Source: Our Fragile X World
| First try | Second try | Never correct | |
|---|---|---|---|
| Doctor visit | 90% | 7% | 3% |
| Risk | 92% | 7% | 1% |
| Duration of study | 92% | 1% | 7% |
| Placebo | 61% | 18% | 21% |
| Randomization | 83% | 7% | 10% |
| Societal benefit | 67% | 26% | 7% |
What does this mean for families?
Individuals with FXS can find information about a clinical trial hard to understand. Our Fragile X World researchers learned that using simple words, repeating information, and giving multiple-choice options can help individuals with FXS make a choice about taking part in a clinical trial. Although some parts of an individual's ability to consent had to do with things that are hard to change (e.g., IQ), there are other characteristics that could be improved. For example, the lower an individual's anxiety, the more able they were to understand information about clinical trials.
Asking questions to check for understanding during the consent process can show researchers where more clarification, education, or support is needed. Families considering a clinical trial for their child can ask researchers to provide supports such as these during the consent or assent process.
Related Resources
Please visit these web sites to learn more about clinical trials:
- Information about informed consent for clinical trials can be found on this website sponsored by the U.S. Food and Drug Administration: https://www.fda.gov/patients/clinical-trials-what-patients-need-know/informed-consent-clinical-trials
- Click on the FAQs and Resources button to find lots of information on participating in clinical trials from the National Fragile X Foundation: https://fragilex.org/our-research/myfxresearch-portal
- This National Institutes of Health (NIH) web site provides information about clinical trials: https://clinicaltrials.gov/ct2/about-studies/learn
Read the full article by Anne Wheeler and colleagues
Source. Wheeler, A. C., Wylie, A., Raspa, M., Villagomez, A., Miller, K., Edwards, A., DeRamus, M., P. S., Appelbaum & Bailey, D. B. (2019). Decisional Capacity for Informed Consent in Males and Females with Fragile X Syndrome. Journal of Autism and Developmental Disorders, 1-23.
